The humble fruit fly has once again taken center stage in a fascinating discovery that could change how we understand organ damage and inflammation. In a groundbreaking study, researchers have identified a peculiar protein that seems to be at the heart of kidney damage in these flies.
The journey began in the lab where scientists, led by Fumiaki Obata, set out to understand why chronic immune activation leads to organ damage. They focused on the fruit fly’s equivalent of kidneys, known as Malpighian tubules (MTs), which play a crucial role in maintaining the fly’s fluid balance and waste excretion. The team used genetic tools to activate an immune pathway called the ‘Imd pathway’ specifically in these tubules, mimicking conditions of chronic inflammation.
What they observed was startling. The flies developed a “bloating” phenotype, their abdomens swelling with excess water, which is a sign of compromised kidney function. They measured the flies’ body water content, finding that those with activated Imd pathways had significantly higher wet weights compared to controls, indicating increased water retention without changes in dry weight. This was not just a physical change; it led to a shortened lifespan and increased vulnerability to high-salt diets, where survival rates plummeted.
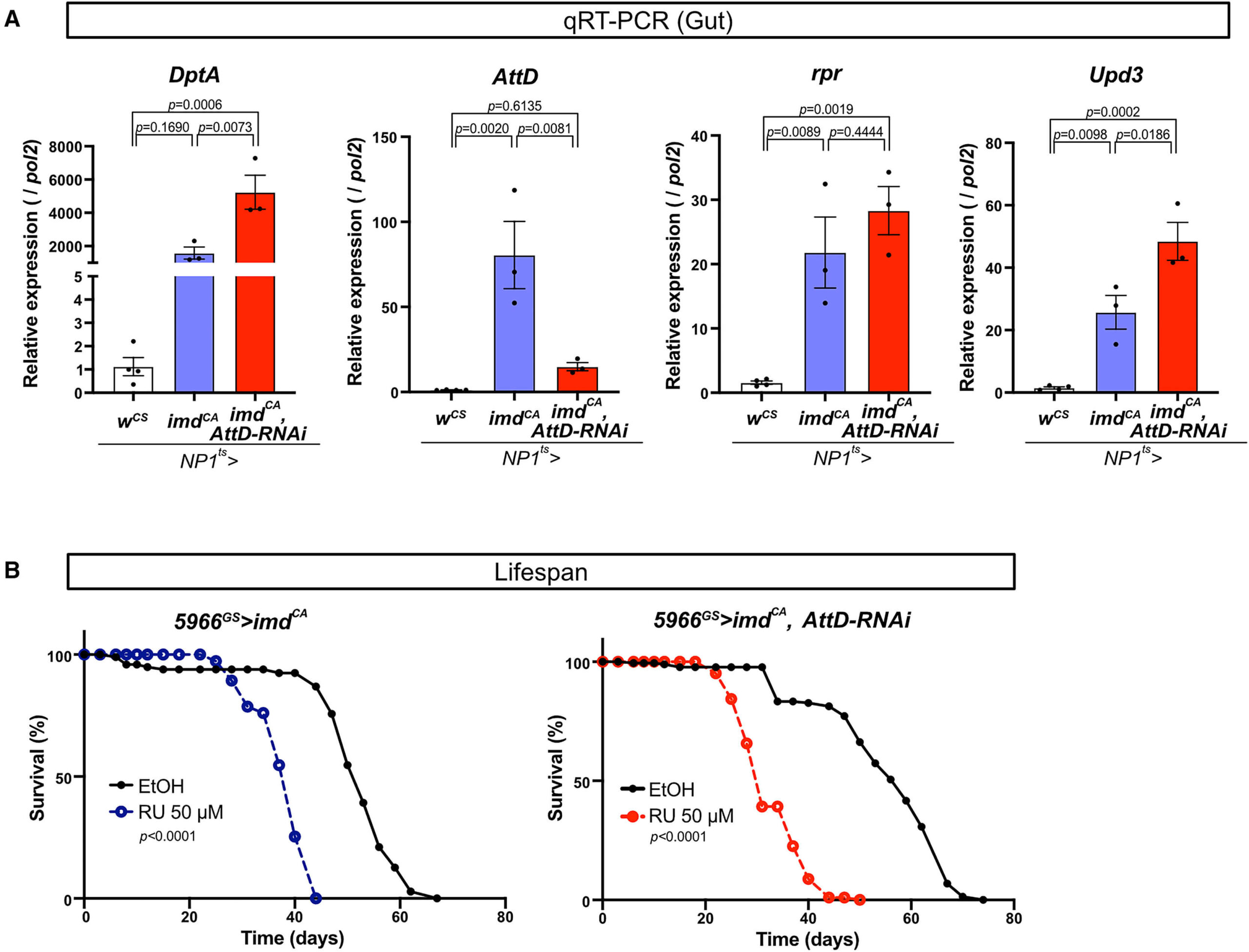
Digging deeper, the researchers conducted a genetic screening to pinpoint which gene was responsible for this damage. They identified Attacin-D (AttD), a protein not like others. Unlike most antimicrobial peptides that are secreted to fight off infections outside the cell, AttD lacks a signal peptide, meaning it stays inside the cell. This unique characteristic made it a prime suspect for causing internal damage.
When they knocked down or mutated the AttD gene, the damage reversed. Flies no longer bloated, their lifespan extended, and their resilience against a high-salt diet improved dramatically. For instance, the survival rate on a high-salt diet, which was around 50% in flies with activated Imd pathways, jumped back to nearly 90% when AttD was knocked down. This wasn’t just about reversing one symptom; the entire array of pathological changes, from cell death in the tubules to systemic effects like increased levels of allantoin (a biomarker for purine levels), were mitigated.
But how does AttD cause such havoc? The study suggests that AttD aggregates inside the cells, possibly forming structures that could disrupt cellular function. They observed AttD forming puncta or clumps within the cells, and in Western blot analyses, they noted that AttD can form dimers or trimers, indicating its potential to oligomerize and perhaps even form pores in organelles like mitochondria, akin to how some proteins damage bacterial membranes.
Interestingly, this damage was specific to the Malpighian tubules. When the researchers looked at other organs like the gut or wing discs, even with Imd pathway activation, AttD did not play a role in the damage observed there. This specificity raises intriguing questions about why and how this protein targets one organ and not another.
Reference
https://www.cell.com/cell-reports/fulltext/S2211-1247(24)01433-5








