In a groundbreaking study, researchers have revealed how the Earth’s mantle turns into a veritable gold factory, which could reshape our understanding of how precious metals are distributed across the planet. Deng-Yang He, along with a team of scientists, has pieced together the puzzle of how subduction zones, where one tectonic plate dives under another, become hotspots for gold concentration.
The journey of gold from the mantle to the crust, where it forms deposits we can mine, has long puzzled geologists. It’s known that subduction zones are key players in this process, as they are the primary regions where mass exchanges between the mantle and the crust occur. However, the exact mechanisms behind the enrichment of gold in these areas were not fully understood—until now.
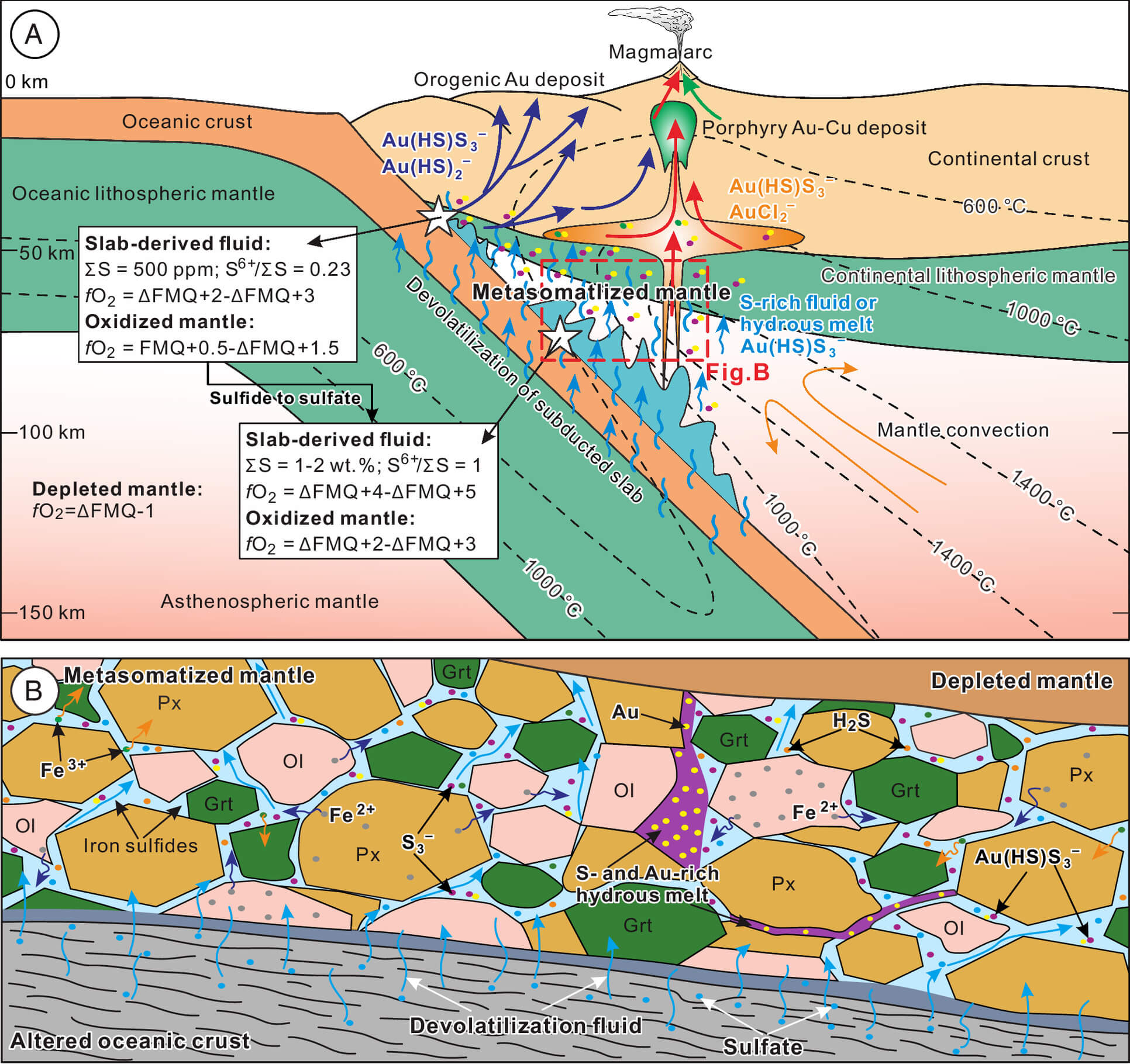
The team, led by He, employed sophisticated thermodynamic simulations to mimic the conditions under the Earth’s surface where subduction happens. Their findings? Sulfur is the unsung hero in this gold saga. When the oceanic crust subducts, it releases water-rich fluids loaded with sulfur. As these fluids infiltrate the mantle wedge above, they bring about a dramatic change.
Here’s where it gets fascinating: the sulfur in these fluids is initially in its oxidized form, largely as sulfate. When these sulfates react with the mantle, they oxidize it, increasing its oxygen fugacity by up to 3 to 4 log units compared to the untouched mantle. This oxidation process is not just a chemical curiosity; it’s the catalyst for gold’s journey.
The magic happens when the sulfate transforms into a less oxidized form, specifically the trisulfur radical ion (S3–). This ion forms a stable, soluble complex with gold, known as Au(HS)S3–, which can carry gold concentrations of several grams per cubic meter of fluid. To put this into perspective, this is more than three orders of magnitude higher than the average gold content in the mantle.
Imagine this like a natural conveyor belt where gold is not just passively sitting in rocks but actively being transported and concentrated by these sulfur-rich fluids. The study quantifies that this process can extract 10 to 100 times more gold from the mantle than would be possible without these fluids, particularly at redox conditions where sulfides and sulfates can coexist.
Kun-Feng Qiu, another key researcher involved, explains, “Our data show that the interaction of these sulfur-bearing fluids with the mantle essentially makes the mantle a gold-rich environment, setting the stage for the formation of gold deposits when these enriched fluids eventually lead to the creation of gold-rich magmas.”
This research doesn’t just stop at explaining how gold gets concentrated; it also sheds light on why some regions of the world are more gold-rich. Subduction zones like those in the Pacific Ring of Fire, where the Earth’s crust is constantly recycled, are places where this natural gold factory is most active.
Reference
He, D., Qiu, K., Simon, A. C., Pokrovski, G. S., Yu, H., Connolly, J. A., Li, S., Turner, S., Wang, Q., Yang, M., & Deng, J. (2024). Mantle oxidation by sulfur drives the formation of giant gold deposits in subduction zones. Proceedings of the National Academy of Sciences, 121(52), e2404731121. https://doi.org/10.1073/pnas.2404731121








