In a groundbreaking exploration of the brain’s molecular symphony, scientists have peeled back the layers to reveal a stunning precursor to neurological disorders.
The study, led by Sameer S. Bajikar and his team, focuses on the MeCP2 protein, crucial for normal brain function. MeCP2 is a well-known player in Rett syndrome, a severe neurological disorder, but its role in adult brain function is less understood. The team embarked on a journey to explore what happens when MeCP2 is suddenly lost in adult mice, wanting to see if initial molecular changes could signal upcoming neurological deficits.
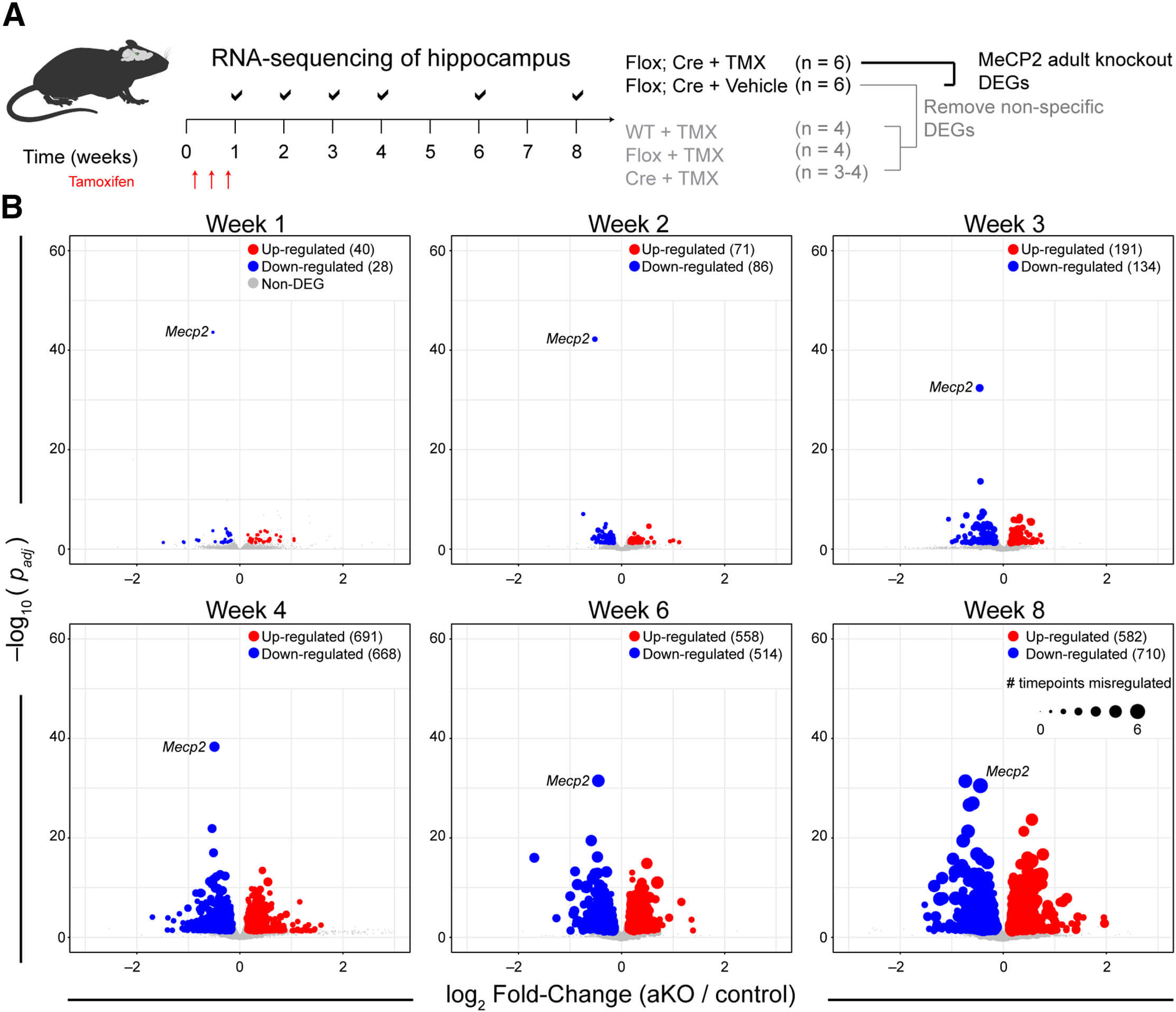
Using a cutting-edge approach called RNA sequencing, they charted the transcriptional landscape of the mouse brain over several weeks following the deletion of MeCP2. Amazingly, they discovered that even before any physical symptoms appeared, there was a significant shift in the expression of a vast array of genes. Within the first week, 68 genes showed altered activity, with 40 genes being upregulated and 28 downregulated. As weeks progressed, this dysregulation spread like wildfire, affecting over a thousand genes by the fourth week.
The researchers meticulously mapped these changes, finding a striking pattern of which genes were affected. They noted that many of these genes are associated with crucial brain functions such as synaptic signaling and neuron development. These transcriptional changes occurred much earlier than any measurable behavioral declines, suggesting they might serve as early warning signals for neurological diseases.
One of the standout revelations from this research was the dynamic nature of chromatin—a complex of DNA and protein that forms chromosomes—surrounding these dysregulated genes. It was observed that there were subtle, yet significant, changes in histone modification, particularly acetylation, that mirrored the gene expression alterations. This finding implies that the chromatin landscape is highly responsive to the presence of MeCP2 and that its absence can lead to profound molecular shifts that tip the scales towards disease.
Huda Y. Zoghbi, a key figure in the study, emphasized the importance of these findings, noting that the early transcriptional changes might inform future diagnostic strategies for neurological disorders. “Understanding these early molecular cues could pave the way for preemptive therapeutic interventions before the onset of severe symptoms,” she remarked.
This research not only advances our understanding of MeCP2’s role in adult brain function but also highlights the broader importance of dosage-sensitive genes in maintaining neural health. The implications are vast, offering potential pathways to explore for early diagnosis and treatment of complex neurological conditions.
Reference
https://doi.org/10.1016/j.neuron.2024.11.006








